i used to believe that the battery capacity is not been near entirely used (by random application) so i a bit "wasted my time" on studding 'em // -- as in fact it turns out the battery keeps it's output "Up" for up to 90% of it's capacity
the processes present at exploitation (not discussed further in this article - just listed)::
0) internal electro mechanics and electrical field set up by load current and env. temperature
1) reduction of the chemistry induced charge
2) chemistry change by discharge
3) re-inducing the charge by present chemistry
i made the series of simple discharge v. time tests
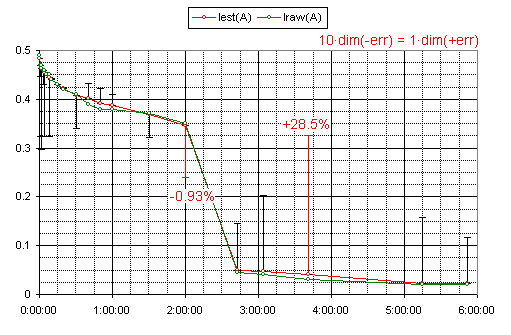
the values for internal resistance and electro-motoric force are derived theoretically(1)
notice! that the experiment recorded just terminal voltage and current levels -- everything else is derived
the legend explained::
C836mAh(%) -- the percentage of "full capacity" -- 836mAh in this case
iC -- 1 (100%) minus C836mAh(%)
E -- electro-motoric force in volts
r20Ω(%) -- internal resistance / 20Ω
Iest(A) -- estimated terminal current in amperes
P(W) --U x I -- external- / diffusing power
Csimple(%) -- R.const C battery alternate (as using capacitor + delimiting resistor inplace of an alkaline)
about the error of the used theoretical model(1)
i speculate that the master fluctuations are due error in device redings -- whatever the case it's not so significant
the E(r) or r(E) dependency(1) is defined from measuring multiple AA cells at their various discharge levels
notice! that there's no time dependency defined
[EOF]




No comments:
Post a Comment